💰 The Investment Thesis
The landscape of drug discovery and development is undergoing a seismic shift, driven by the relentless advancement of Artificial Intelligence.
Breakthroughs like Google DeepMind's AlphaFold, now celebrating five years of unparalleled impact in protein structure prediction, have fundamentally altered our understanding of biological mechanisms.
This isn't just an incremental improvement; it's a paradigm shift that collapses timelines and costs associated with identifying drug targets and designing novel therapeutics. Parallel progress in bio-inspired computing, exemplified by the development of artificial neurons that mimic real brain cell behavior, promises to unlock entirely new computational architectures capable of processing complex biological data at an unprecedented scale and nuance. 🧬💻🤖
The core investment thesis hinges on the convergence of these capabilities:
Accelerated Target Identification & Validation: AI can rapidly sift through vast biological datasets to pinpoint disease-causing proteins and pathways with higher accuracy than traditional methods.
De Novo Drug Design: Moving beyond screening, AI can generate novel molecular structures optimized for specific targets, including difficult-to-drug proteins. This significantly expands the chemical space for potential therapies.
Personalized Medicine at Scale: By integrating omics data (genomics, proteomics, metabolomics) with clinical information, AI can predict individual responses to treatments, leading to highly effective, personalized therapies and reducing trial failures.
Operational Efficiency & Cost Reduction: From automating contract analysis (as demonstrated by Condé Nast and Myriad Genetics with AWS Bedrock) to streamlining R&D workflows, AI slashes operational overheads, making drug development faster and cheaper.
Enhanced AI Safety & Control: As OpenAI highlights with "confessions to keep language models honest" and AWS demonstrates with "fine-grained access control," the critical need for secure, controllable, and ethical AI deployments in sensitive biotech applications creates a ripe market for specialized infrastructure and governance solutions.
This confluence creates a 'disrupt or be disrupted' scenario. Incumbent pharmaceutical giants, while eventually adapting, are shackled by legacy systems and cultural inertia. This creates an enormous window of opportunity for agile, AI-native biotech startups to capture significant market share by building foundational platforms and pipelines that leverage these new capabilities from day one.
The initial focus should be on areas where AI provides an insurmountable advantage: novel target discovery, small molecule/biologic design, and optimizing clinical trial design. The venture capital community must move decisively to back these foundational disruptors.
🚀 The Disruptor's Playbook: Entry Strategy
To enter and dominate this market, a new player must leverage AI's core strengths to solve a critical bottleneck in the existing drug development pipeline. The strategy involves creating an AI-native Drug Discovery & Optimization Platform.
Minimum Viable Product (MVP): "Target-to-Lead AI Synthesizer" 🎯
This MVP would focus on rapidly identifying novel, high-affinity small molecule drug candidates for a single, well-validated, but difficult-to-drug protein target. The target could be implicated in a rare disease or an indication with high unmet medical need where traditional methods have failed.
MVP Core Features:
AI-Driven Target Analysis: Ingest publicly available (and proprietary, if accessible) structural, functional, and genomics data related to the chosen protein target. Use advanced AI models (e.g., AlphaFold-like structural prediction, generative models for binding site identification) to deeply characterize the target's druggability and identify cryptic binding pockets. 🔍
Generative Molecular Design:
Employ deep generative models (e.g., variational autoencoders, GANs, diffusion models trained on vast chemical libraries) to de novo design millions of small molecules. These molecules would be optimized for binding affinity, specificity, and initial ADMET (Absorption, Distribution, Metabolism, Excretion, Toxicity) properties in silico. 🧪
High-Throughput Virtual Screening & Optimization:
Rapidly screen the generated molecules against the target protein using molecular dynamics simulations and binding energy prediction algorithms. Iterate the generative design based on these computational results, continuously optimizing lead candidates. ⚡
Automated Synthesis Planning:
For the top ~100 predicted lead candidates, integrate AI-driven retrosynthesis tools to propose viable synthetic routes, assessing chemical feasibility and cost-effectiveness. ⚙️
Cloud-Native & Secure Infrastructure:
Built entirely on scalable cloud platforms (e.g., AWS Bedrock for LLMs, specialized HPC for simulations), ensuring fine-grained access control and data security, addressing enterprise concerns. 🔒
Steps to Rule the Market and Displace Incumbents:
Hyper-Specialization & Speed:
Focus intensely on proving superiority for a particularly challenging target where current pharma has limited success. Rapidly move from target identification to a few high-quality, synthesizable lead compounds within 6-12 months – a timeline impossible with traditional methods. This speed is the ultimate disruptor. 💨
Strategic Partnerships:
Collaborate with academic labs or small biotechs for initial in vitro and in vivo validation. Avoid building a full wet lab initially to maintain agility and capital efficiency. This de-risks the drug candidates and builds credibility. 🤝
Data Moat Construction:
Prioritize continuous data feedback loops. Every in silico prediction and subsequent wet lab validation (even from partners) refines the AI models. This creates an ever-improving data moat, making the platform increasingly powerful and difficult for competitors to replicate. 💾
Expand 'Druggable' Space:
After initial success, systematically tackle other 'undruggable' targets, expanding the frontier of therapeutic possibilities. This demonstrates broad applicability and value beyond a single success story. 🌟
Leverage Open-Source & Community:
Contribute to open-source initiatives (like the AWS Generative AI IDP Accelerator) where appropriate, attracting talent and building a reputation, while protecting core proprietary algorithms and data. 🌐
Acquisition Bait:
Develop a pipeline of promising pre-clinical candidates, making the company an attractive acquisition target for larger pharma companies desperate for innovation and speed, or to go public with a validated platform and diverse pipeline. 💰
This integrated approach, driven by cutting-edge AI and a focus on speed and efficiency, will not merely compete but fundamentally redefine how drugs are discovered, establishing a new market leader. 👑
📊 Projected P&L (Year 1-3)
Projection for the "Target-to-Lead AI Synthesizer" platform, focusing on initial service model and licensing potential. Assumes successful proof-of-concept and early partnerships.
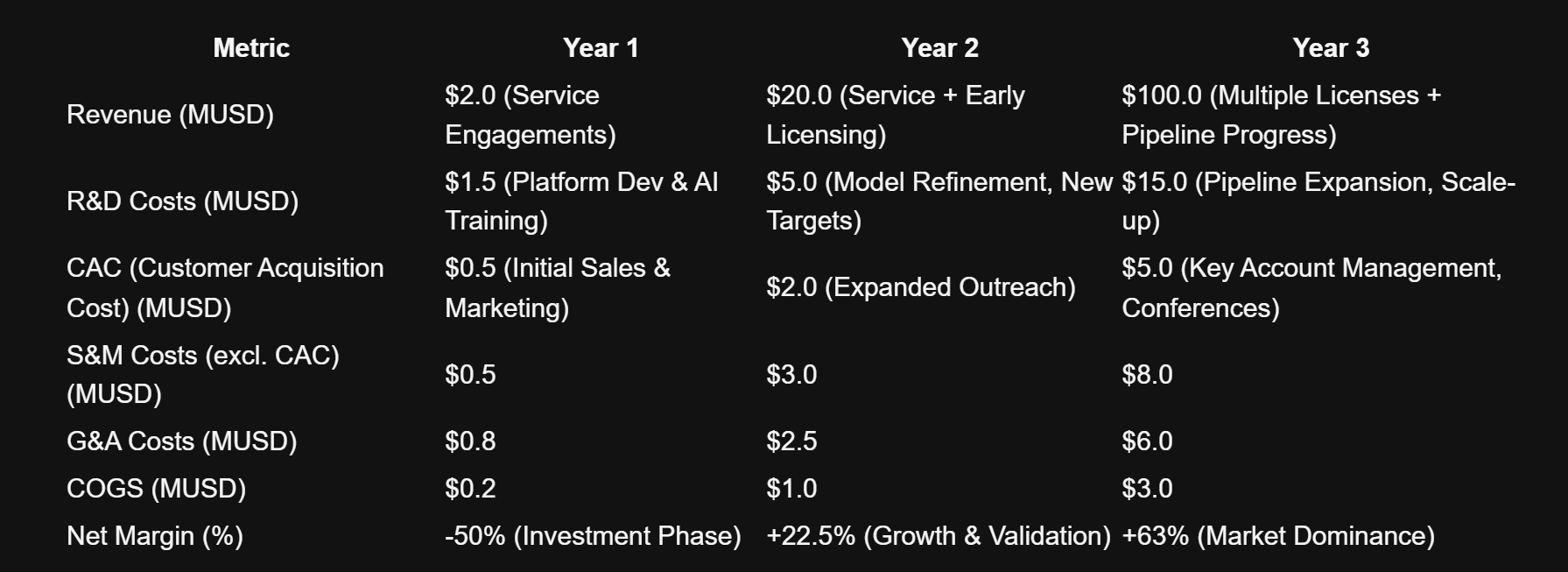
Note: These projections are aggressive and rely on successful execution, rapid validation, and strategic partnerships/licensing deals. Early-stage biotech carries significant inherent risk, but the AI-driven advantage is expected to accelerate these milestones.
⚠️ Risk Analysis
While the opportunity is immense, several risks must be meticulously managed:
Regulatory Hurdles:
Drug development is heavily regulated. Demonstrating the reliability and interpretability of AI-generated candidates to regulatory bodies (FDA, EMA) will be crucial. This forms a significant moat for those who master it. 🏛️
Technical Moats & IP:
While open-source AI tools exist, proprietary data sets for validation and highly optimized, specialized AI architectures will be key differentiators. Competitors will attempt to replicate successes. Robust IP protection on novel algorithms and generated molecular entities is paramount. 🔒
Talent Scarcity:
The convergence of deep AI expertise and biological domain knowledge is rare. Attracting and retaining top-tier interdisciplinary talent will be a continuous challenge and a significant competitive advantage. 🧠
Data Quality & Bias:
"Garbage in, garbage out" applies acutely to AI. The quality and representativeness of training data for generative models are critical. Biases in datasets could lead to ineffective or even harmful drug candidates. Diligent data curation and ethical AI practices are non-negotiable. 📉
Incumbent Resistance & Integration:
Large pharma companies have vast resources. While slow to innovate, they may acquire promising startups or invest heavily in their own AI capabilities. The disruptor's strategy must include a clear path for either acquisition or sustained, independent growth. 🏢
Computational Scale:
Running complex molecular simulations and training advanced generative AI models is computationally intensive and expensive. Ensuring access to cutting-edge hardware and cloud resources (like AWS's highly optimized ML services) at scale is essential. 💸
Mitigating these risks through strategic legal counsel, continuous R&D, strong talent acquisition, and an adaptive business model will be key to translating this alpha into market dominance. The prize is redefining medicine itself. 🏆